The World Health Organisation (WHO) has licensed the new oral polio vaccine type 2 and granted it prequalification. The objective is to completely eradicate polio and The Medical Research Council Unit The Gambia at the London School of Hygiene & Tropical Medicine (MRCG at LSHTM) has been central in this.
Below is the full text of the press release issued by the MRC.
[Banjul, The Gambia] 26 February 2024; The Medical Research Council Unit The Gambia at the London School of Hygiene & Tropical Medicine (MRCG at LSHTM) welcomes the news that novel oral polio vaccine type 2 (nOPV2) has been licensed and that is has been granted prequalification by the World Health Organization (WHO). This is a critical step forward towards polio eradication globally.
According to Polio Global Eradication Initiative, since 1988, the global polio eradication effort has seen a remarkable 99.9% reduction in wild polio cases. Yet, challenges persist, particularly with vaccine-derived poliovirus outbreaks. Contributing to addressing this, MRCG at LSHTM played an important role in trials leading to the licensure of nOPV2. The phase 3 trial of the vaccine, which generated vital data was completed at MRCG at LSHTM. The Unit has also published important data on the use of nOPV2 when given to children in a national campaign in The Gambia.
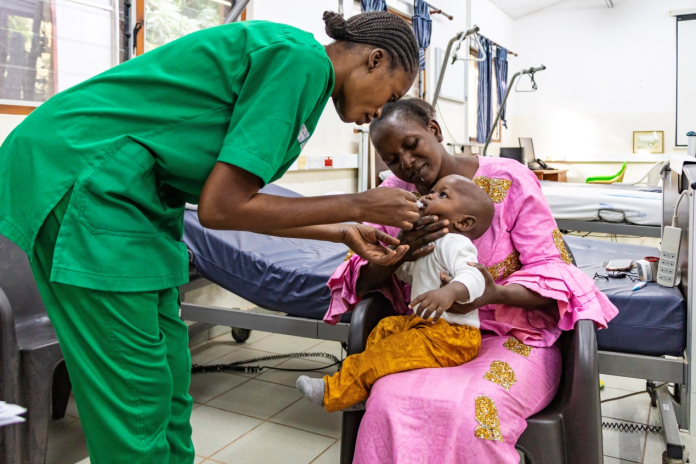
nOPV2 was genetically engineered to be more stable and reduce the risk of reversion events which rarely result in vaccine-derived poliovirus outbreaks. Given the urgent need for an oral polio vaccine to ensure eradication efforts remained on track, nOPV2 became the first vaccine globally to receive WHO’s Emergency Use Listing in November 2020, based on early trial data. The trial conducted at MRCG at LSHTM found that nOPV2 is safe and induces a strong immune response to the poliovirus in infants and young children. The trial took place in The Gambia where 2,345 infants (18-52-weeks-old) and 600 young children (aged 1-5 years) were vaccinated between February and October 2021.
After two doses of nOPV2, analysis of blood samples indicated that over 80% of infants and children gained new protection against polio infection. Overall, over 90% of infants and children were protected at the end of the study. Other data also confirm that nOPV2 is more genetically stable when given to infants and children so is much less likely to cause vaccine-derived polio. The trial found no safety concerns with nOPV2.
Approximately 1 billion doses of nOPV2 administered across 35 countries will protect millions of children from polio-related illness and paralysis.