By Nelson Manneh
The Medicines Control Agency on Saturday, 4 October 2024 recalled two medicinal products (Salbutamol Oral Solution and Plasmodial tablets) from the Gambian Market.
The Agency, during its post-market surveillance, discovered two medicinal products in circulation that have changed colour due to degradation despite not yet expired and therefore, are considered substandard.
Salbutamol Oral Solution BP 2mg/5ml, 100ml manufactured by RONALD PHARMACEUTICALS PVT LTD in India, imported and distributed in The Gambia by Serenba Pharmaceuticals Co Ltd with Manufacture date June 2022, Expiry date May, 2025 and Batch number 220656. The product has changed colour from clear solution to yellowish or orange colour with some crystals in it.
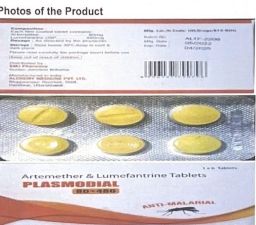
PLASMODIAL 80+ 480 (Artemether 80mg & Lumefantrine 480mg, 6Tablets/strip), manufactured by ALCHEMY MEDICINE PVT LTD in India, imported and distributed in The Gambia by EMJ Pharmacy with Manufacture date May 2022, Expiry date April, 2025 and Batch number ALTF-2206. The tablet has changed colour with dark spots possibly due to the growth of mould.
“MCA has started recalling the affected batches of these two medicinal products with immediate effect and therefore, the public is warned against the use of these products despite are not expired,” the Agency outlined.
Patients in possession of these products should return them to the pharmaceutical outlets or health facilities where they acquired the products.
“All Pharmaceutical Outlets and Health Facilities in possession of these products must surrender them to the MCA Office or its Inspectors in the field doing the recall,” the Agency stated.
Mr Essa Maranah, the Executive Director of the Medicine Control Agency, said the two medicinal products (Salbutamol Oral Solution BP 2mg) his agency is recalling did not change colour at the time of their importation.
“All these medicinal products were good and up to date, they are yet to expire. When our officers went to the field they realized that these drugs have changed colour due to degradation despite that they are not yet expired and therefore, are considered substandard,” he said.
ED Maranah said it was probably due to the temperature in which they were stored.
“We want the public to know that these products are recalled and should not be consumed. This is not because they failed their test during importation but because they changed colour and normally when drugs change colour it means they are no longer good for consumption,” he said.